Assume the reaction A + B → C + D proceeds to equilibrium. Calculate the equilibrium concentration of D at  given that the starting concentrations of A and B are 2M and that △G° for the reaction is
given that the starting concentrations of A and B are 2M and that △G° for the reaction is 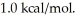
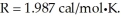
Definitions:
Japan
An island country located in East Asia, known for its rich history, cultural heritage, technological advancements, and economic power.
Physical Control
The ability to maintain influence over one's physical actions and environment, often used in the context of health or personal discipline.
Insecure Attachment
A type of attachment style characterized by difficulty in forming secure and trusting relationships with others, often resulting from early childhood experiences.
Secure Attachment
A healthy form of attachment characterized by trust in the availability and support of attachment figures, fostering confidence and independence.
Q9: The nurse is reviewing activities to assess
Q15: A bicyclic alkane contains 12 carbon atoms.
Q19: A patient is admitted after being bitten
Q25: Draw a mechanism that shows how the
Q30: Which of the following compounds would reduce
Q30: How many distinct dichlorination products can result
Q68: Provide the major organic product of the
Q84: Does one expect ΔS° in a propagation
Q99: Which of the following statements is a
Q107: The H-bonds formed in the tertiary structure