Consider the reaction (CH3) 3CBr + CH3CH2OH → 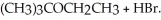 Experimentally one finds that if the concentration of (CH3) 3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
Experimentally one finds that if the concentration of (CH3) 3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
Definitions:
Assessment Focus
The central point or primary goal that guides the evaluation or examination of a specific issue or entity.
Service Technology
Technologies designed to facilitate the delivery of services, enhancing efficiency and customer experience.
Organizational Resources
Assets, capabilities, and processes that an organization utilizes to function effectively and achieve its objectives.
Service Delivery
The process of providing services to customers, clients, or citizens in an efficient and satisfactory manner.
Q16: Which nursing activities are examples of independent
Q16: The nurse manager of a care area
Q23: Which of the following amino acids does
Q36: Amino acids have _.<br>A) high melting points
Q57: Phospholipids are generally produced from the phosphatidate
Q77: Which compound has the smaller bond dissociation
Q106: Which of the following is a carbene?<br>A)
Q114: Provide the IUPAC name of (CH<sub>3</sub>CH<sub>2</sub>)<sub>3</sub>CH.
Q115: Which of the following statements is (are)
Q127: What would you start with when making