What is the outcome of the following reaction? 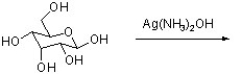
Definitions:
Neutrons
Subatomic particles found within the nucleus of an atom, having no electric charge and a mass slightly larger than that of protons.
Atomic Number
The number of protons in the nucleus of an atom, which determines the chemical properties of an element and its place in the periodic table.
Mass Number
The total number of protons and neutrons in the nucleus of an atom, representing the atom's mass.
Electronegativity
A chemical property that describes the tendency of an atom to attract a shared pair of electrons towards itself in a covalent bond.
Q25: Would you expect the carbonyl carbon of
Q41: By which single reaction can benzene be
Q42: The CCN bond angle in acrylonitrile (CH<sub>2</sub>=CHCN)
Q49: Explain why the pKa of acetylene is
Q70: A polymer with a high degree of
Q77: Show how the following reaction may be
Q77: The structure of amygdalin is shown below.
Q80: The molecule shown below contains _ pi
Q102: Derivatives of the compound shown below are
Q109: Provide the structure of the major organic