Multiple Choice
According to the 1,3-butadiene structure below, which positions would be best to place methoxy groups to yield the most reactive dimethoxy-1,3-butadiene isomer in the Diels-Alder reaction? 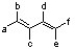
Definitions:
Related Questions
Q29: Rank the following bonds in order of
Q32: In electrophilic aromatic substitution reactions a bromine
Q69: Provide the structure of the major organic
Q73: The reagent which converts a carbonyl group
Q82: Draw a mechanism for the following reaction.
Q86: Provide the major organic product of the
Q102: Derivatives of the compound shown below are
Q105: What diene and dienophile would react to
Q108: Provide the proper IUPAC name for CH<sub>3</sub>CHBrCH<sub>2</sub>COCH<sub>2</sub>CHO.
Q128: In a Diels-Alder reaction, what is the