What pattern is the fourth step of this reaction? 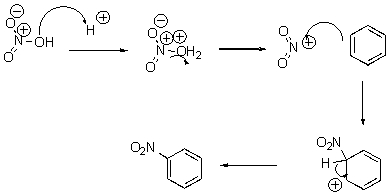
Definitions:
Sigma Bonds
The strongest type of covalent chemical bond, formed by the direct overlap of atomic orbitals, with the electrons shared in an area centered on a line running between the nuclei.
Pi Bonds
Covalent chemical bonds formed by the side-to-side overlap of atomic orbitals, allowing for electron sharing in double and triple bonds.
Hybridization
A concept in chemistry that describes the mixing of atomic orbitals into new hybrid orbitals, suitable for the pairing of electrons to form chemical bonds.
Atom
The basic unit of a chemical element, consisting of a nucleus of protons and neutrons, with electrons orbiting this nucleus.
Q1: Is the following nucleophile strong or weak?
Q8: Why would the following reaction sequence not
Q16: What type of cleavage does the following
Q22: The regioselectivity and stereospecificity in hydrohalogenation of
Q31: Provide an IUPAC name for the following
Q35: Is the following more likely a nucleophile
Q45: Provide the systematic IUPAC name for BrCH<sub>2</sub>CH<sub>2</sub>C≡CCH<sub>2</sub>CH<sub>3</sub>.<br>A)1-Bromo-3-hexyne<br>B)6-Bromo-3-hexyne<br>C)1-Bromo-2-hexyne<br>D)6-Bromo-4-hexyne<br>E)1-Bromo-4-hexyne
Q64: In the following reaction,how many H's do
Q83: Identify the electrophilic atom in the following
Q106: Which of the following is a valid