Exhibit 30-1 to Answer the Following Question(s),consider the π Molecular Orbitals of Orbitals
Exhibit 30-1
To answer the following question(s) ,consider the π molecular orbitals of a conjugated diene shown below: 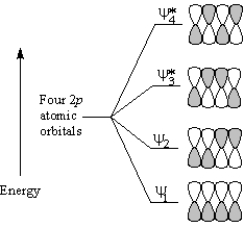
-Refer to Exhibit 30-1.Which molecular orbital of a conjugated diene contains two nodes between nuclei?
Definitions:
Double Bonds
Chemical bonds between two atoms involving four bonding electrons instead of the usual two, making the bond stronger and the structure more rigid.
Lewis Structure
A graphical representation of the valence electrons of atoms within a molecule, showing how they are arranged and shared to form bonds.
Chloromethane
A colorless, flammable gas with a faintly sweet odor, used in the production of silicones.
Lewis Structure
A diagrammatic representation of the bonding between atoms of a molecule and the lone pairs of electrons in the molecule.
Q4: The nurse is caring for a postoperative
Q7: The nurse is calculating the dosage of
Q8: A client who takes an intermediate- or
Q11: Which of the following is deoxyadenosine? A
Q15: Clinical experimentation occurs in four stages. The
Q18: What hemiacetal would form from the internal
Q19: Which of the following dipeptides is L−Cys−L−Ala?<br>A)<br><img
Q21: A client is ordered to receive potassium.
Q29: Draw the structure of polymer formed in
Q29: Draw:<br>diisopropylamine