What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol∙K) . 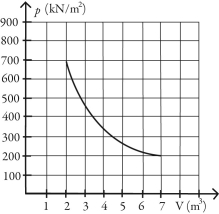
Definitions:
Delegated Medical Functions
Tasks assigned to healthcare professionals by physicians that are within the scope of the professionals' training.
Safety Checklist
A list of items or criteria to be reviewed to ensure that an environment or procedure meets safety standards.
Surgical Site
The area on the body where surgery is performed, which is prepared, sterilized, and often marked before the procedure begins.
Double Gloving
The practice of wearing two layers of gloves for protection, typically used in medical and hazardous material handling to reduce the risk of contamination or puncture.
Q6: The acceleration of an object as a
Q7: A transverse wave is traveling on a
Q7: If a certain sample of an ideal
Q11: A lawn roller in the form of
Q12: The cube of insulating material shown in
Q14: A ball drops some distance and gains
Q19: A 2.00-kg block of ice at 0.00°C
Q22: The near point of a person's uncorrected
Q34: Two long straight parallel lines, #1 and
Q76: A uniform solid cylindrical log begins rolling