The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of nitrogen is 14 g/mol. 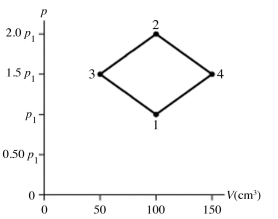
Definitions:
Retailer
A business entity that sells goods to consumers for personal or household use, varying from small shops to large chain stores.
Hybrid Strategy
A competitive strategy that combines elements of both cost leadership and differentiation to create a unique market position and appeal to a broad range of customers.
Near-Sourcing
The practice of transferring a business operation closer to where products are sold or where the business is located, often to reduce lead times and costs.
Stockout
A situation in which the demand or requirement for an item cannot be fulfilled from the current inventory or stock.
Q1: A metal bar is hanging from a
Q4: A 2.00 kg piece of lead at
Q6: In the figure, a very small toy
Q9: A diffraction grating has 300 lines per
Q14: A 50-cm<sup>3</sup> block of wood is floating
Q15: A radiating body originally has a Kelvin
Q21: Two strings of identical material and radius
Q24: The focal lengths of the objective and
Q40: The angular momentum of a system remains
Q71: A 4.50-kg wheel that is 34.5 cm