The figure shows the pV diagram for a certain thermodynamic process. In this process, 1500 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 9.00 x 104 Pa. If the volume of the system increases from 0.020 m3 to 0.050 m3, calculate the change in internal (thermal) energy of the system. If the internal (thermal) energy change is nonzero, be sure to indicate whether this energy change is positive or negative. 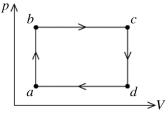
Definitions:
Semantic Memory
A branch of long-term memory involved in the storage of general world knowledge, facts, concepts, and meanings.
Short-Term Memory
The component of the cognitive system that is responsible for temporarily holding information available for processing.
Chunking
A memory technique involving organizing information into familiar, manageable units or groups.
Flashbulb Memories
Vivid, detailed memories of significant or shocking events that feel as if they were imprinted onto the brain like a photograph.
Q3: A 5.00-kg object moves clockwise around a
Q12: Jupiter completes one revolution about its own
Q18: The two water reservoirs shown in the
Q23: A thin beam of laser light of
Q43: A wave pulse traveling to the right
Q46: The walls of an ice chest are
Q62: A rock is under water in a
Q73: In the figure, point P is at
Q77: When an object is solely under the
Q81: A potter's wheel, with rotational inertia 46