A cylinder contains 1.2 moles of ideal gas, initially at a temperature of 116°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 6.4 x 105 Pa on the gas. The gas is cooled until its temperature has decreased to 27°C For the gas 
and the ideal gas constant 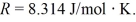
(a) Find the work done by (or on) the gas during this process. Is the work done by or on the gas?
(b) What is the change in the internal (thermal) energy of the gas during this process?
(c) How much heat is transferred to (or from) the gas during this process? Does this heat flow into or out of the gas?
Definitions:
Internet Transactions
Commercial or financial transactions conducted electronically on the internet.
Acceptance
The act of agreeing to the terms of an offer, thereby forming a contract.
Offer
A tentative promise to do something if another party consents to do what the first party requests.
Telephone
A telecommunications device that allows two or more users to conduct a conversation when they are too far apart to be heard directly.
Q1: A mass M is attached to an
Q7: At most, how many bright fringes can
Q11: A +7.00 μC point charge and -9.00
Q23: When a rocket is traveling toward a
Q33: A steel container, equipped with a piston,
Q38: A sphere is constructed of two concentric
Q39: A tennis ball bounces on the floor
Q44: A 2.0-m string is fixed at both
Q45: Find the net work done by friction
Q82: When a solid melts<br>A) the temperature of