Multiple Choice
The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3. Find the value of V3. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the atomic weight of helium is 4.0 g/mol. 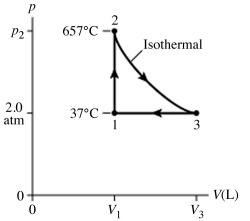
Definitions:
Related Questions
Q2: The length and width of a rectangle
Q12: Which of the following foods has the
Q13: At a distance of 2.00 m from
Q30: A 2.00-kg object traveling east at 20.0
Q32: A compression, at a constant pressure of
Q36: A 15-g bullet is shot vertically into
Q37: A cat runs along a straight line
Q44: A glass plate whose index of refraction
Q56: The figure shows the position of an
Q92: Two identical balls are thrown directly upward,