Multiple Choice
The figure (not to scale) shows a pV diagram for 1.8 g of helium gas (He) that undergoes the process 1 → 2 → 3. Find the value of V3. The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the atomic weight of helium is 4.0 g/mol. 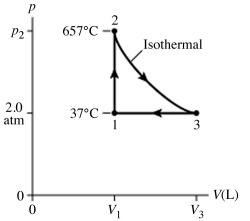
Definitions:
Related Questions
Q4: A 3.2-L volume of neon gas (Ne)
Q7: A transverse wave is traveling on a
Q18: A cube at 100.0°C radiates heat at
Q19: If the acceleration of an object is
Q20: An 8.0-g bullet is shot into a
Q25: Light is incident normally from air onto
Q27: A 2.0 g bead slides along a
Q28: Waves travel along a 100-m length of
Q42: What is the critical angle for light
Q48: A 120-kg refrigerator, 2.00 m tall and