The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 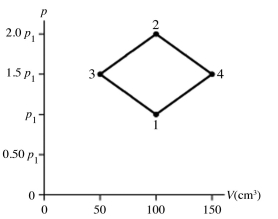
Definitions:
Market Price
The present cost at which a service or asset is available for purchase or sale.
Par Value Common Stock
The nominal or face value assigned to common shares in the charter of a corporation, which has little relation to the market value.
Paid-in Capital
The amount of money shareholders have invested in a company in exchange for equity, excluding any earnings retained by the company.
Market Value
The ongoing rate at which a commodity or service is offered for buying or selling in the market.
Q3: A 5.00-kg object moves clockwise around a
Q13: A rock is suspended from a scale
Q31: A certain crying baby emits sound with
Q41: A diffraction grating is to be used
Q43: A machine part is vibrating along the
Q48: An ideal gas in a balloon is
Q51: Two identical stones are dropped from rest
Q59: What is the radius of a sphere
Q61: A container with rigid walls is filled
Q98: A student slides her 80.0-kg desk across