Explain why the following reaction does not occur to any significant extent respect when the reactant is treated with bromine in the presence of acetic acid.Atoms other than carbon and hydrogen are labeled.
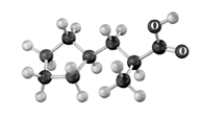
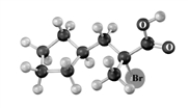 Reactant Product
Reactant Product
Definitions:
Examine If Open
A control logic instruction used in PLC programming to check if a specific condition or input is false or "open" before executing subsequent instructions.
Output Energize
The process of activating an output device or circuit, thereby enabling it to perform its intended function, such as turning on a light or motor.
Input Energize
refers to the condition in electrical or electronic circuits where an input signal or power source is applied, activating a component or process.
Dividend
A portion of a company's earnings that is distributed to shareholders, typically in the form of cash or additional stock.
Q3: Give a one sentence definition of replication.
Q11: Consider the following titration curve for an
Q12: Refer to Exhibit 9-5.The key intermediate in
Q14: Refer to Exhibit 22-1.Choose the most acidic
Q14: Refer to Exhibit 23-10.Show how you might
Q19: Refer to Exhibit 26-1.Draw the condensed structure
Q25: Refer to Exhibit 19-1.The substance formed on
Q25: The following product is a simplified penicillin
Q63: _ a β-1,4'-glycoside<br>A)starch<br>B)cellulose<br>C)<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4944/.jpg" alt="_ a β-1,4'-glycoside
Q69: Identifies 12 complementary colors for text,background,accents,and links.