Exhibit 10-2
To answer the following question(s) consider the reaction below: 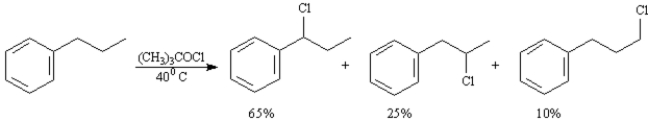
-A)When propylbenzene reacts with tert-butylhypochlorite three monochlorinated products are formed in the ratios indicated.Calculate a reactivity order for each type of hydrogen atom in propylbenzene.
B)The reaction of propylbenzene with tert-butylhypochlorite proceeds by a radical substitution pathway.Draw the structure of the radical intermediate leading to each product.
C)Based on your answers to the two questions above explain why (1-chloropropyl)benzene is the major product of this reaction.
Definitions:
Industrialized Countries
Nations that have undergone industrialization, characterized by a significant development in manufacturing, infrastructure, and a shift from agricultural to industrial and service-based economies.
Supreme Court
The highest judicial body in a country, responsible for interpreting the constitution and laws, and resolving legal disputes.
Former Strikers
Individuals who have previously participated in strikes and have since returned to work, often with experiences and perspectives that influence their approach to labor relations.
Bargaining Power
The capacity of one party in a negotiation to influence the terms of agreement due to their resources, alternatives, or strategic advantage.
Q6: Choose the best reagent for carrying out
Q7: Consider the two energy diagrams below for
Q12: _ Intermediate in the elimination-addition mechanism of
Q14: What type of hybridization is exhibited by
Q17: Write the complete stepwise mechanism for the
Q18: Ethyl acetate and 2-butene-1,4-diol both have the
Q27: Refer to Exhibit 9-3.How many degrees of
Q28: Refer to Exhibit 22-6.Explain the product ratio
Q32: In the initial stage,vomiting results in:<br>A) metabolic
Q34: Aklomide,2-chloro-4-nitrobenzamide,is an ingredient in veterinary antibacterial preparations.Propose