Exhibit 2-2
Calculate the formal charges on the indicated atoms in each compound below. 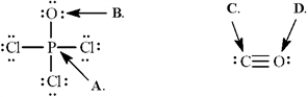
-Refer to Exhibit 2-2.The formal charge on carbon (C) is ______.
Definitions:
Hydrogen Ions
Positively charged atoms of hydrogen, significant in acid-base chemistry and pH balance in biological systems.
Acidic
Having a pH less than 7, indicating the presence of a higher concentration of hydrogen ions (H+) in solution.
Hydrophobic
Characterized by a tendency to repel water; substances that do not mix with water.
Buffered
A solution's capacity to resist changes in pH when acids or bases are added.
Q5: Draw the E2 mechanism of the reaction
Q7: _ is a cyclic alkane with two
Q9: A physical constant that is the quantitative
Q16: Which of the following can be detected
Q19: Which of the following would be has
Q20: The two structures show below represent: <img
Q22: Which of the following statements applies to
Q28: Draw the mechanism of the alkylation of
Q28: The pathologic change associated with scleroderma is:<br>A)
Q28: Explain your energy order from lowest to