A hydrogen atom makes a downward transition from the 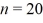 state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
Definitions:
Economic Development
The process by which a nation improves the economic, political, and social well-being of its people, often through increased industrial activity, infrastructure development, and workforce improvement.
Railroads
Transportation systems consisting of tracks and trains, used for the movement of goods and passengers.
Ganglion Cells
Types of neurons located near the inner surface of the retina of the eye, which process visual information and send it to the brain.
Axons
Long, thread-like structures in neurons that transmit electrical impulses away from the neuron's cell body, crucial for neural communication.
Q5: A small dust particle of mass 7.90
Q7: The copy constructor from the base class
Q15: An electron in a hydrogen atom is
Q18: What is the output of the following
Q27: In order to do automatic type conversion
Q28: Most applications that use a stack will
Q31: Light in air is initially traveling parallel
Q41: When an LRC series circuit is driven
Q51: Which is the correct way to tell
Q60: Which of the following statements about β<sup>+</sup>