A hydrogen atom makes a downward transition from the 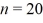 state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
Definitions:
Expanded Accounting Equation
An extension of the basic accounting equation that includes components such as owner's equity, retained earnings, and dividends.
Beginning Balances
The balances of accounts at the start of a new accounting period, carried over from the end of the prior period.
Transactions
Financial events that affect the financial position of a company, measurable in terms of changes in assets, liabilities, and equity.
Statement Of Owner's Equity
A financial statement that shows the changes in the ownership interest of a company over a period of time.
Q8: A 10-inch telescope (25.4 cm in diameter)is
Q11: In a particular case of Compton scattering,a
Q20: A series LRC circuit consists of a
Q24: Which part of the ADT tells the
Q27: A negatively charged particle is moving to
Q30: A member function that allows the user
Q38: A closed,circular loop has a counter-clockwise current
Q45: When monochromatic light illuminates a grating with
Q48: If a base class has public member
Q52: A beam of electrons is accelerated through