What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K) . 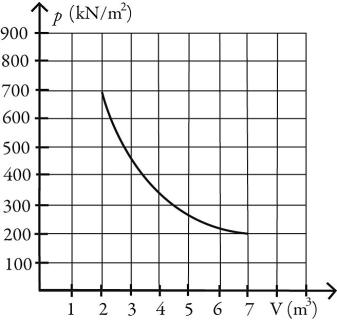
Definitions:
Small Production Batches
Manufacturing process involving the production of goods in small quantities, allowing for flexibility and customization.
Lean Manufacturing
A systematic method for waste minimization within a manufacturing system without sacrificing productivity, focusing on adding value and reducing non-value-adding activities.
Eliminating Waste
The process of removing non-value-adding activities in a business to improve efficiency.
Inventory Levels
Inventory levels refer to the quantity of goods and materials on hand at any given time within a business, which is crucial for meeting customer demand without overstocking.
Q8: Consider a solenoid of length L,N windings,and
Q14: If a current of 2.4 A is
Q19: The weight of spaceman Speff at the
Q31: A 5.0-μC point charge is placed at
Q34: An uncharged 30.0-µF capacitor is connected in
Q35: You want to insert an aluminum rod,which
Q43: A 910-kg object is released from rest
Q43: A galvanometer has an internal resistance of
Q44: A thin copper rod that is 1.0
Q57: A very long,hollow,thin-walled conducting cylindrical shell (like