What is the change in entropy of 10.0 moles of ideal monatomic gas that reversibly undergoes the isothermal expansion shown in the figure? The ideal gas constant is R = 8.314 J/(mol ∙ K) . 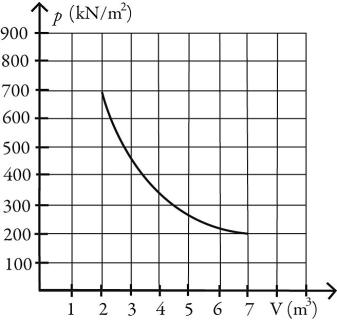
Definitions:
Antipyretics
Medications used to reduce fever or lower body temperature.
Lumbar Puncture
A medical procedure where a needle is inserted into the spinal canal to collect cerebrospinal fluid for diagnostic testing.
Bacterial Meningitis
An infection of the membranes covering the brain and spinal cord, often resulting in fever, headache, and a stiff neck, caused by bacteria.
Q3: In the figure,a small spherical insulator of
Q21: The figure (not to scale)shows a pV
Q21: A standing wave is oscillating at 690
Q22: A simple harmonic oscillator has an amplitude
Q27: As you stand by the side of
Q34: An astronaut is standing on the surface
Q36: A frictionless simple pendulum on Earth has
Q42: If we double only the amplitude of
Q58: What is the maximum length of a
Q66: A vertical wire carries a current vertically