What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 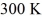 ? The Boltzmann constant is 1.38 × 10-23 J/K.
? The Boltzmann constant is 1.38 × 10-23 J/K.
Definitions:
Fiscal Policy
The use of government spending and tax policies to influence economic conditions, including economic growth, inflation, and unemployment.
Monetary Policy
A strategy by which a central bank controls the supply of money in an economy, often targeting interest rates to achieve economic stability or growth.
Federal Reserve
The central banking system of the United States, responsible for setting monetary policy, regulating banks, and ensuring financial stability.
Social Security Act
A U.S. law enacted in 1935 that created a system of transfer payments in which younger, working people support older, retired people.
Q5: A hot air balloon has a volume
Q8: A 1.0-μF and a 2.0-μF capacitor are
Q12: A dipole with a positive charge of
Q19: A 3.0-μC positive point charge is located
Q20: What is the minimum magnitude of an
Q21: The root-mean-square speed (thermal speed)of the molecules
Q23: The two water reservoirs shown in the
Q44: The figure shows a pV diagram for
Q45: Four resistors are connected across an 8-V
Q55: A coil of 160 turns and area