What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 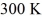 ? The Boltzmann constant is 1.38 × 10-23 J/K.
? The Boltzmann constant is 1.38 × 10-23 J/K.
Definitions:
Hawthorne Studies
A series of studies conducted in the early 20th century that examined how work environment and conditions affect worker productivity, highlighting the importance of social factors.
Human Relations
The study and practice of improving interaction and understanding between people in a workplace or social contexts.
Workplace Relations
The dynamics and interactions between employees and employers, including communication, teamwork, and conflict resolution.
Behavioral Problems
Refers to actions or conduct that are inappropriate or problematic, often causing concern in social, educational, or professional settings.
Q8: You are driving along a highway at
Q15: A point charge Q is located a
Q16: A point charge Q of mass 8.50
Q25: A heavy stone of mass m is
Q29: An ideal gas is kept in a
Q30: The figure shows the displacement y of
Q41: A quantity of ideal gas requires 800
Q51: Each plate of an air-filled parallel-plate air
Q57: A very light 1.00-m wire consists of
Q76: Ions having equal charges but masses of