A fixed amount of ideal gas goes through a process abc.In state a,the temperature of the gas is 152°C,its pressure is 1.25 atm,and it occupies a volume of 0.250 m3.It then undergoes an isothermal expansion to state b that doubles its volume,followed by an isobaric compression back to its original volume at state c.(Hint: First show this process on a pV diagram.)The ideal gas constant is 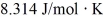 ,and 1.00 atm = 1.01 × 105 Pa.
,and 1.00 atm = 1.01 × 105 Pa.
(a)How many moles does this gas contain?
(b)What is the change in the internal energy of the gas between states a and b?
(c)What is the net work done on (or by)this gas during the entire process?
(d)What is the temperature of the gas in state c?
Definitions:
Credit Balance
An accounting term describing a situation where the credits in an account exceed the debits, often indicating a liability or revenue.
Liability Account
An accounting record that represents an entity's obligations to pay debts owed to other entities in the future.
Horizon Problems
Challenges faced by management in making decisions that benefit short-term performance at the expense of long-term gains, often influenced by the tenure of managers or external pressures.
Dividend Retention
The strategy of reinvesting company profits instead of distributing them to shareholders as dividends to fuel future growth.
Q6: The point charge at the bottom of
Q13: A sample of an ideal gas is
Q14: When an object is solely under the
Q18: A charge Q is uniformly spread over
Q23: The two water reservoirs shown in the
Q23: A 1.2-kg spring-activated toy bomb slides on
Q25: The figure shows a 2.0-cm diameter roller
Q33: A 20.0-kg uniform plank is supported by
Q34: A 10.0-kg shell is traveling horizontally to
Q38: At what temperature would the root-mean-square speed