If you add 700 kJ of heat to 700 g of water at 70.0°C,how much water is left in the container? The latent heat of vaporization of water is 2.26 × 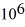 J/kg and its specific heat is 4190 J/(kg ∙ K) .
J/kg and its specific heat is 4190 J/(kg ∙ K) .
Definitions:
Labor and Capital
Describes the economic and social relationship between workers (labor) and the owners of businesses (capital).
Caesar's Column
A novel by Ignatius L. Donnelly published in 1890, depicting a future society in which inequalities and economic injustices lead to a revolution in the streets of New York City.
Walter Rauschenbusch
A Christian theologian and key figure in the Social Gospel movement, advocating for social reform based on Christian ethics.
Q5: For the circuit shown in the figure,determine
Q10: A cold trap is set up to
Q14: An uncharged 1.0-μF capacitor is connected in
Q21: A galvanometer coil having a resistance of
Q25: Two long parallel wires carry currents of
Q30: An electron is released from rest at
Q38: A man-made satellite of mass 6105 kg
Q40: A board that is 20.0 cm wide,5.00
Q42: If we double only the amplitude of
Q44: Water flows in the horizontal pipe shown