How many grams of ice at -13°C must be added to 711 grams of water that is initially at a temperature of 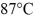 to produce water at a final temperature of
to produce water at a final temperature of 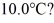 Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Assume that no heat is lost to the surroundings and that the container has negligible mass.The specific heat of liquid water is 4190 J/kg ∙ °C and of ice is 2050 J/kg · °C.For water the normal melting point is 0.00°C and the heat of fusion is 334 × 103 J/kg.The normal boiling point is 100°C and the heat of vaporization is 2.26 × 106 J/kg.
Definitions:
No-Par Stock
Shares issued without a par value, which means their face value is not fixed in the stock certificate, enabling more flexibility in setting the share prices.
Stated Value
The nominal value assigned to no-par value stock by the company's board of directors, used for accounting purposes.
Legal Capital
The minimum amount of capital that a company is required to maintain as per law, to protect creditors and ensure that the company remains solvent.
Corporate Form
A legal structure of a business organization that is recognized as a separate legal entity, often characterized by limited liability for its owners and issuance of stocks.
Q13: A horizontal wire carries a current straight
Q18: A barge is 15.0 m wide and
Q32: Calculate the current through a 10.0-m long
Q32: An ideal gas is compressed in a
Q40: Two positive point charges +4.00 μC and
Q41: Equal but opposite charges Q are placed
Q41: A quantity of ideal gas requires 800
Q44: The exterior of a supersonic airplane is
Q52: The four identical capacitors in the circuit
Q72: A point charge Q moves on the