An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 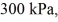 the initial volume is
the initial volume is 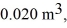 and the initial temperature is
and the initial temperature is 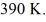 The final pressure is
The final pressure is 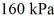 and the final temperature is
and the final temperature is 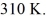 The change in the internal (thermal) energy of the gas is closest to
The change in the internal (thermal) energy of the gas is closest to
Definitions:
Promising Young Companies
Emerging businesses that show potential for significant growth and success in their industry.
Mezzanine Financing
A hybrid form of capital typically used in late-stage financing composed of debt and equity, ranking between senior debt and equity in terms of claim on assets.
Principle Amount
The original sum of money borrowed in a loan or the initial amount of investment before interest or profit.
Equity
The value of an ownership interest in property, including shareholders' equity in a corporation, representing the residual value to shareholders after debts and liabilities have been settled.
Q7: One very small uniformly charged plastic ball
Q12: A 2.00-kg block of ice at 0.00°C
Q18: By how many newtons does the weight
Q21: A galvanometer coil having a resistance of
Q24: The figure shows two unequal point charges,q
Q36: At atmospheric pressure,45 moles of liquid helium
Q43: The small piston of a hydraulic lift
Q44: If the mass of the earth and
Q44: The exterior of a supersonic airplane is
Q84: A thermally isolated system is made up