A cylinder contains 1.2 moles of ideal gas,initially at a temperature of 116°C.The cylinder is provided with a frictionless piston,which maintains a constant pressure of 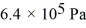 on the gas.The gas is cooled until its temperature has decreased to 27°C.For the gas
on the gas.The gas is cooled until its temperature has decreased to 27°C.For the gas
CV = 11.65 J/mol · K,and the ideal gas constant R = 8.314 J/mol ∙ K.
(a)Find the work done by (or on)the gas during this process.Is the work done by or on the gas?
(b)What is the change in the internal (thermal)energy of the gas during this process?
(c)How much heat is transferred to (or from)the gas during this process? Does this heat flow into or out of the gas?
Definitions:
Nk Cells
Natural Killer cells are a type of white blood cell that plays a significant role in the host-rejection of both tumors and virally infected cells.
Fight-Or-Flight Reaction
A physiological response to perceived harmful events, attacks, or threats to survival, priming the body for either confronting or avoiding the danger.
Blood Pressure
The pressure of circulating blood on the walls of blood vessels, which is one of the principal vital signs.
Immune Activity
The biological processes and reactions of the immune system in detecting and fighting off pathogens.
Q1: A pipe that is 20.0 m long
Q4: A 2.0-m string is fixed at both
Q18: A silver wire has a cross sectional
Q20: What is the minimum magnitude of an
Q21: A satellite in a circular orbit of
Q23: A silver wire with resistivity <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg"
Q28: A frictionless pendulum released from 65 degrees
Q44: A certain electric furnace consumes 24 kW
Q44: If we double only the mass of
Q79: The process shown in the pV diagram