What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is R = 8.314 J/mol ∙ K = 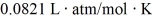 .
.
Definitions:
Good-Faith Contract
An agreement between parties characterized by mutual respect, honesty, and trust, often informal and based on verbal commitments.
Goal Statement
A concise description of the objectives a person, group, or organization aims to achieve.
General Task
A broad or comprehensive duty or activity that may encompass various specific jobs or responsibilities.
Baseline Measure
An initial set of data or observations that serve as a starting point for comparison with later data.
Q5: There must be equal amounts of mass
Q9: A 1.5-kg mass attached to an ideal
Q10: Ekapluto is an unknown planet that has
Q28: A 5.00-kg object moves clockwise around a
Q41: Dust particles are pulverized rock,which has density
Q42: A coal-fired plant generates 600 MW of
Q46: In the figure,a mass of 31.77 kg
Q51: Two harmonic sound waves reach an observer
Q58: A 20.0-kg uniform door has a width
Q72: When a fixed amount of ideal gas