The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container.The temperature T1 of the gas in state 1 is 79°C.What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 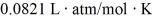 ,and the ATOMIC weight of nitrogen is 14 g/mol.
,and the ATOMIC weight of nitrogen is 14 g/mol. 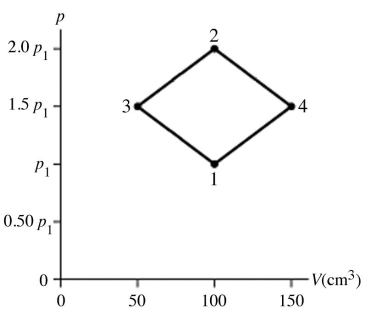
Definitions:
Social Security Payments
Government transfers paid to eligible individuals, such as the retired, disabled, and survivors, aimed at providing a basic level of financial security.
Private Pensions
Retirement plans offered by private sector employers, unions, or other organizations to provide income to employees after retiring.
GDP
Gross Domestic Product represents the sum value of all products and services generated within a country's boundaries during a certain timeframe.
Consumption of Services
The action of using services offered in the economy, which can include healthcare, education, and entertainment among others.
Q13: The figure shows a pV diagram for
Q18: A heavy stone of mass m is
Q19: In the figure,charge <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4470/.jpg" alt="In the
Q22: A multiloop circuit is shown in the
Q28: Standing waves of frequency 57 Hz are
Q29: A thin 2.00-m string of mass 50.0
Q40: A board that is 20.0 cm wide,5.00
Q66: The weight of a car of mass
Q92: An 80-g aluminum calorimeter contains 380 g
Q95: The pV diagram shown is for 7.50