Which would be true about the following reaction? M + N 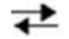 Y + Z
Y + Z
Definitions:
Process Controls
The methods and procedures implemented to monitor and manage business processes to ensure efficient and effective operation and to meet goals and standards.
Line Units
Departments or teams directly involved in the core operations or primary purpose of an organization.
Division of Labor
The allocation of different parts of a manufacturing process or task to different individuals in order to improve efficiency.
Coordinating Mechanisms
Methods or systems used within an organization to align activities, tasks, and functions towards achieving common objectives.
Q2: Which of the following would NOT be
Q5: Which of the following statements regarding higher
Q6: A high-amplitude, spike-wave EEG pattern is characteristic
Q17: First messengers may bind to a membrane
Q29: In general, polar molecules diffuse more rapidly
Q31: The composition of the fluid bathing the
Q53: What category do hair cells in the
Q54: The difference in electrical charge between two
Q59: Adequate folate intake for neural tube development
Q77: In cardiac muscle cells the release of