Consider the reaction: H2CO3 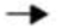 CO2 + H2O + 4 kcal/mol. Which of the following is TRUE?
CO2 + H2O + 4 kcal/mol. Which of the following is TRUE?
Definitions:
Geometric Tolerance
The maximum permissible deviation in shape and location for a geometric element.
Characteristic Symbol
A symbol used in engineering drawings to represent the type of geometric characteristic that is being controlled by a geometric tolerance.
Symmetry
A characteristic where an object or design is evenly proportioned and balanced, such that it can be divided into matching halves by one or more axes.
Straightness
A condition where an element or line follows a true, unbending course, often used to describe the accuracy of a manufactured part.
Q1: A presynaptic synapse:<br>A) is a synapse between
Q7: The shape of a molecule may change
Q9: The Na+/K+-ATPase carrier transports sodium ions out
Q11: The greater the concentration difference of a
Q22: What is the term for the segments
Q25: What will happen if a normal cell
Q48: The fiber type intermediate between the two
Q58: Which of the following statements regarding the
Q83: An action potential in a neuronal membrane
Q87: In the visual pathway providing sensory action