The standard reduction potential for ubiquinone (Q or coenzyme Q) is 0.045 V, and the standard reduction potential (E'°) for FAD is -0.219 V. Using these values, show that the oxidation of FADH2 by ubiquinone theoretically liberates enough energy to drive the synthesis of ATP. The Faraday constant, 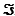 , is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
, is 96.48 kJ/V · mol. G'° for ATP synthesis is +30.5 kJ/mol.
Definitions:
Incentive
Something that motivates or encourages someone to do something.
Biological Deficiency
A condition where an organism lacks a substance or element necessary for its growth, maintenance, or functioning.
Achievement
Successfully reaching a goal through effort, skill, or courage, often recognized by some form of acknowledgment or reward.
Money
A medium of exchange, in the form of coins and banknotes, used to facilitate transactions for goods and services.
Q2: Show the reaction that limits the rate
Q2: Suppose you found an overly high level
Q9: Under what circumstances does the bifunctional protein
Q17: The standard reduction potentials (E'°)for the
Q18: In "C<sub>4</sub>" plants of tropical origin,the first
Q25: Explain how mutations in the following proteins
Q25: Lipoprotein particles in human blood do not
Q38: Which one of the following sugar phosphates
Q44: Show the reaction catalyzed by glycogen synthase.
Q53: Why is the drug acyclovir effective against