Both pyridine and pyrrole shown below have a lone pair of electrons on their nitrogen capable of acting as a base.Which compound would you expect to be the stronger base.Why? 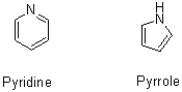
Definitions:
Liquidated Damages
A contractual provision that specifies a predetermined sum to be paid if a party breaches the agreement.
Reasonably Related
A legal standard used to determine if the connection between two concepts or actions is logical or justified, often in the context of regulatory or employment actions.
Compensatory Damages
Monetary awards given to a plaintiff to compensate for losses, injury, or harm suffered due to the defendant's actions.
Impossibility of Performance
A legal doctrine where a party is released from a contract because events have occurred making it objectively impossible to perform the contract's obligations.
Q4: Alkynes cannot be a part of conjugated
Q6: Which of the following would be an
Q10: Thomas Hobbes believed that people gave up
Q13: Which of the following would be an
Q21: In which of the following ways must
Q26: Which of the following statements about Japan's
Q27: Max Weber's three forms of political legitimacy
Q37: Ascription occurs when<br>A)one ethnicity develops negative stereotypes
Q52: Which of the following compounds is considered
Q58: What would be the expected product from