Both pyridine and pyrrole shown below have a lone pair of electrons on their nitrogen capable of acting as a base.Which compound would you expect to be the stronger base.Why? 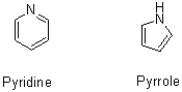
Definitions:
Current Stock Price
The price at which a specific stock is trading on the stock market at any given time.
EPS Growth Rate
EPS Growth Rate measures the annual rate at which a company's earnings per share (EPS) has grown over a specified time period.
Investors Rate of Return
The percentage of profit or loss made on an investment over a specific period, measuring the efficiency of an investment.
Appropriate Discount Rate
The interest rate used to discount future cash flows of an investment, reflecting the risk level and the time value of money.
Q2: Single-member district systems are<br>A)electoral systems in which
Q7: Which of the following events is widely
Q17: How many atoms are part of the
Q20: Which of the following statements describes a
Q25: What would be the kinetic product of
Q31: Since the major economic downturn of the
Q38: Which side will the equilibrium of the
Q40: What is the torsional angle between the
Q63: Which of the following represents the
Q75: Lithium aluminum hydride reductions of ketones can