Essay
Propose a synthetic scheme to obtain the following product.Show all intermediate structures. 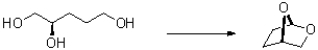
Definitions:
Related Questions
Q4: A diene is activated with electron withdrawing
Q16: In an E2 elimination reaction,the leaving group
Q19: Which of the following is the stronger
Q29: Which of the following best categorizes sites
Q38: Which conditions would push the forward direction
Q51: Propose a mechanism for the following transformation.
Q52: S<sub>N</sub>2 reactions lead to a racemic mixture
Q54: Electron-donating substituents on aromatic rings act as
Q60: Photochemical electrocyclic reactions always involve disrotatory movement
Q74: What is the product of the reaction