An unknown compound X has the empirical formula C3H6O and a molecular ion in its mass spectrum at 116.Compound X shows no IR absorption at 3200-3600 cm-1 but shows a peak at 1700 cm-1.The 1H NMR spectral data of X is shown below.What is the structure of compound X? 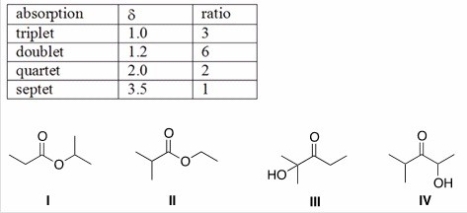
Definitions:
Right Hemisphere
The half of the brain that controls the left side of the body, and is often associated with creativity and spatial abilities.
Specialized Abilities
Unique skills or talents that an individual may excel in, often requiring specific training or innate capability distinct from general competencies.
Broca's Area
A region in the frontal lobe of the dominant hemisphere of the brain with functions linked to speech production.
Frontal Area
The frontal area, often referring to the frontal lobes of the brain, plays a critical role in decision making, personality expression, and moderating social behavior.
Q8: An unknown compound has the formula of
Q19: Which of the labeled protons absorbs furthest
Q20: Upon hydrolysis of cellulose,what sugar is formed?<br>A)Sucrose<br>B)Maltose<br>C)Lactose<br>D)Glucose
Q21: The new organization of cloth production included
Q22: What is an intramolecular Claisen reaction called?<br>A)Michael
Q24: Which class of compounds listed below does
Q24: Which of the following is D-sorbose? <img
Q36: Where were the first two universities?<br>A)Oxford and
Q37: Which of the indicated protons absorbs further
Q51: The consolidation of royal authority in France