An unknown compound X has the empirical formula C3H6O and a molecular ion in its mass spectrum at 116.Compound X shows no IR absorption at 3200-3600 cm-1 but shows a peak at 1700 cm-1.The 1H NMR spectral data of X is shown below.What is the structure of compound X? 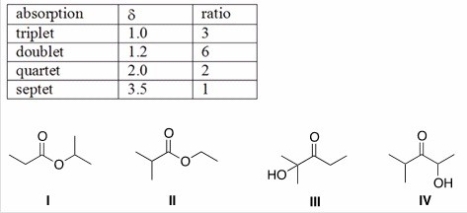
Definitions:
Unemployed Persons
Individuals actively seeking employment but unable to find work.
Inflation
A general increase in prices and fall in the purchasing value of money over a period of time.
Salesperson
An individual who sells goods or services to customers, often responsible for negotiating prices, offering product insights, and closing sales.
Retired Couple
Two individuals in a partnership who have stopped working permanently, often relying on savings, pension, or government benefits for income.
Q6: What is (are)the basic subunits of terpenes?<br>A)Cyclohexane<br>B)Triacylglycerols<br>C)Isoprene<br>D)Carbonyls
Q9: One of the most formidable and powerful
Q10: To the religious tensions that contributed to
Q20: Summarize the expansion of the Ottoman Empire
Q25: Which of the following pairs of carbohydrates
Q33: During the 14<sup>th</sup> century, education in Italy
Q44: Vulcanization is the process of cross-linking polymer
Q48: Modern science indicates that the first human
Q50: How many p molecular orbitals are present
Q59: Which Egyptian deity was considered the creator,