What is the structure of a compound of molecular formula C10H14O2 that shows a strong IR absorption at 3150 ¾2850 cm-1 and gives the following 1H NMR absorptions: 1.4 (triplet,6H) ; 4.0 (quartet,4H) ; and 6.8 (singlet,4H) ppm? 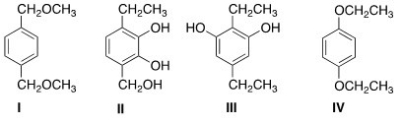
Definitions:
Bankruptcy Proceedings
The legal process through which individuals or businesses unable to meet their financial obligations seek relief from their debts.
Payment Plan
A payment plan is an agreement to pay off an owed amount over a designated period, through specified number of installments.
Order Of Relief
A court order that provides a debtor protection from creditors under bankruptcy proceedings, initiating the automatic stay.
Preferential Payment
A payment made by an insolvent debtor that gives preferential treatment to one creditor over another.
Q10: Select the appropriate sequence of reactions to
Q13: What is the missing reagent in the
Q14: What is the product of the nucleophilic
Q28: What kind of reaction does the conversion
Q38: Which of the following bases are strong
Q38: Would this crossed Aldol reaction work well?
Q38: What is the major alkene formed when
Q42: What is the correct classification of the
Q47: Will acetone be completely deprotonated by potassium
Q48: What would happen if a mixture of