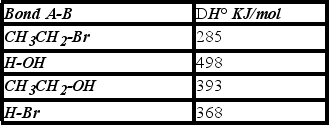
Using the bond dissociation energies given,calculate DH° for the following reaction. 
Definitions:
Shares Outstanding
The total number of shares of stock that are currently owned by shareholders, including restricted shares owned by company insiders.
Reacquired
Refers to stock or securities that a company buys back from its shareholders.
Originally Issued
Refers to securities or financial instruments that are sold for the first time to investors.
Stated Value
The nominal value of a share of stock as determined by the issuing company, which may differ from its market value.
Q7: What reagents are necessary to perform the
Q9: A packaging plant is running a filling
Q31: The conversion of acetyl chloride to methyl
Q34: Which C=O functional group is present in
Q37: Give the IUPAC name for the following
Q39: What is the IUPAC name of the
Q45: Identify the monomer used to make the
Q48: What is the major product of the
Q63: What is the molecular formula of a
Q68: Which of the following is the appropriate