Which of the following compounds would be expected to be more soluble in hexane (C6H14) ? 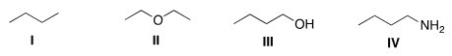
Definitions:
Non-Value-Added
Activities or processes that do not add value to a product or service from the customer's perspective, potentially leading to unnecessary costs.
Value-Added
The enhancement a company gives its product or service before offering the product to customers, which can differentiate a company in the market, increase product value, and command a higher price.
Design Engineering
The branch of engineering focused on the design, development, and testing of products, systems, or structures, ensuring they meet specified requirements.
External Failure Cost
Costs incurred when a product or service fails to meet quality standards after it is delivered to the customer, including returns, repairs, and warranty claims.
Q9: In the United States,most freight is shipped
Q21: What is the product of the following
Q30: As technology and cultures evolve,the best approach
Q31: Which C=O functional group is present in
Q36: Rank the following compounds in order of
Q36: Task X is expected to take three
Q42: What is the major organic product obtained
Q44: What is (are)the starting material(s)in the reaction
Q46: The reaction of tert-butyl bromide,(CH<sub>3</sub>)<sub>3</sub>CBr,with ethanol affords
Q49: Which of the K<sub>eq</sub> corresponds to the