Multiple Choice
How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 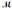 = 84.02 g/mol) ? HCl(aq) + NaHCO3(s) → NaCl(s) + H2O(l) + CO2(g)
= 84.02 g/mol) ? HCl(aq) + NaHCO3(s) → NaCl(s) + H2O(l) + CO2(g)
Understand the role of dopamine in schizophrenia's positive symptoms.
Differentiate between chronic and acute forms of schizophrenia.
Recognize brain abnormalities associated with schizophrenia.
Understand the biopsychosocial model in explaining psychological disorders.
Definitions:
Related Questions
Q7: Given: H<sub>2</sub>O(l) → H<sub>2</sub>O(s) ΔH° = -6.02
Q8: Which of the following statements best defines
Q18: The ground state electron configuration of a
Q31: Identify and explain factors that drive food
Q34: Household sugar, sucrose, has the molecular formula
Q45: The FM station KDUL broadcasts music at
Q45: The oxidation and coordination numbers of cobalt
Q47: Why does the variety of foods available
Q50: Who proposed the principle that states that
Q79: A sample of water is heated at