How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 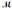 = 84.02 g/mol) ? HCl(aq) + NaHCO3(s) → NaCl(s) + H2O(l) + CO2(g)
= 84.02 g/mol) ? HCl(aq) + NaHCO3(s) → NaCl(s) + H2O(l) + CO2(g)
Definitions:
Made in the U.S.A.
A label indicating that a product has been manufactured or substantially transformed in the United States.
Cooling Off Rule
A federal regulation allowing consumers to cancel certain types of sales or contracts within three days for a full refund.
Rescind Contract
To rescind a contract means to legally cancel or terminate the agreement, returning the parties to the positions they held before entering into the contract.
Do Not Call Registry
A national list managed by the U.S. government where individuals can register their telephone numbers to restrict unsolicited telemarketing calls.
Q9: Select the correct formula for a compound
Q15: A nutritious diet should provide enough of
Q32: Which of the following electron configurations is
Q35: A system that undergoes an adiabatic change
Q46: When the following redox equation is balanced
Q59: Sodium tripolyphosphate is used in detergents to
Q64: Which of the lines on the figure
Q65: Which relationship or statement best describes ΔS°
Q68: "A diamond is forever" is one of
Q88: Select the correct name and chemical formula