Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium chloride and iron. 3Mg(s) + 2FeCl3(s) → 3MgCl2(s) + 2Fe(s) A mixture of 41.0 g of magnesium ( 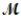 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 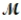 = 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
Definitions:
Income Statement
A financial document that shows a company's revenue, expenses, and profits over a specific period, providing insight into its operational efficiency.
Financial Metrics
Quantitative measures used to assess the financial health, performance, and condition of a business, aiding in decision-making and strategy formulation.
Profit-Leverage Effect
A financial principle indicating that a decrease in operating costs can have a more significant impact on profits than an equivalent increase in sales revenue.
Purchase Spend
Purchase spend refers to the total amount of money a company expends on acquiring goods and services necessary for its operations.
Q20: The orientation in space of an atomic
Q21: Consider the following structures (1 and 2
Q34: "The volume of an ideal gas is
Q49: For a chemical reaction to be non-spontaneous
Q52: What is the name of Na<sub>2</sub>O?<br>A) disodium
Q71: Which one of the following statements about
Q73: Which one of the following elements is
Q76: In forming ions of the first series
Q82: Which one of the following has the
Q84: Sodium chlorate is used as an oxidizer