Tetraphosphorus hexaoxide ( 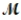 = 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) → P4O6(s)
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) → P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
Definitions:
Necessaries
Goods or services that are essential for maintaining a person's health, education, comfort, or welfare.
Reasonable Value
The fair or market value of a service or item, determined by considering what a willing buyer would pay and a willing seller would accept.
Parents Responsible
An obligation or duty of parents to take care of, make decisions for, and financially support their children.
Torts
A branch of civil law dealing with situations where one's negligence or intentional act causes harm or loss to another, leading to legal liability.
Q3: What are the main mineral sources of
Q10: Which relationship or statement best describes ΔS°
Q21: At very high pressures (~ 1000 atm),
Q39: Calculate q when 28.6 g of water
Q45: The oxidation and coordination numbers of cobalt
Q52: 15.0 g of ice cubes at 0.0°C
Q54: Calculate the ΔH°<sub>rxn</sub> for the following reaction.
Q67: Convert a gas pressure of 485 cmHg
Q89: In the modern periodic table, the order
Q91: Which of the following compounds is covalent?<br>A)