Magnesium reacts with iron(III) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3Mg(s) + 2FeCl3(s) → 3MgCl2(s) + 2Fe(s)
A mixture of 41.0 g of magnesium ( 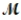 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 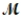 = 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
= 162.2 g/mol) is allowed to react. What mass of magnesium chloride = 95.21 g/mol) is formed?
Definitions:
Mediation Services
Professional services offered to resolve disputes between parties through the intervention of a neutral third-party mediator.
Campus Overcrowded
A situation where the number of students or individuals on a university or college campus exceeds the available space and resources.
Legal Aid
Assistance provided to people unable to afford legal representation and access to the court system.
Savings Account
A bank account that earns interest, offering a secure place for an individual to store their money.
Q3: A 0.850-mole sample of nitrous oxide, a
Q6: Which one of the following is not
Q12: Which one of the following groups does
Q13: In covalent bond formation, the potential energy
Q13: Consider the following octahedral complex structures, each
Q26: A compound of bromine and fluorine is
Q30: An isotope with a high value of
Q62: The Cu<sup>2+</sup> ion has 1 unpaired electron.
Q65: How many grams of oxygen gas will
Q66: Radioactive decay follows zero-order kinetics.