Tetraphosphorus hexaoxide ( 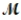 = 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) → P4O6(s)
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) → P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
Definitions:
Email Overload
The state of receiving more emails than can be reasonably read or responded to.
Sales Message
Communication designed to persuade potential customers to purchase a product or service.
Application Message
A message sent during the application process, typically covering interest in a position or responding to a job opening.
Central Selling Feature
The most appealing or distinctive attribute of a product or service that is emphasized in marketing.
Q4: Which of the following elements has the
Q12: Terephthalic acid, used in the production of
Q12: Which of the following ions is most
Q38: What is the name of P<sub>4</sub>Se<sub>3</sub>?<br>A) phosphorus
Q53: Select the classification for the following reaction.
Q64: Which of the following is the empirical
Q71: Barium sulfate is used in manufacturing photographic
Q72: The molecular formula of a compound provides
Q74: Positron decay and electron capture have the
Q91: Consider the reaction of iodine with manganese