Consider the figure that shows ΔG° for a chemical process plotted against absolute temperature. 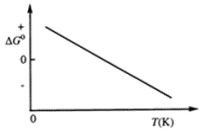 Which one of the following is an incorrect conclusion, based on the information in the diagram?
Which one of the following is an incorrect conclusion, based on the information in the diagram?
Definitions:
Nervous
Relating to the nervous system, which is the network of nerve cells and fibers that transmits nerve impulses between parts of the body.
Haversian Canals
Microscopic tubes or channels within the bone that contain blood vessels and nerves; part of the bone's vascular and innervation system.
Canaliculi
Small, channel-like structures in bones that allow for communication and nutrient exchange between osteocytes.
Lacunae
Small cavities or spaces within tissues, particularly in bone and cartilage, where cells reside.
Q5: Which of the following transition elements can
Q11: Which one of the following statements about
Q25: The reaction of nitrogen with oxygen to
Q30: When the reaction A → B +
Q63: K<sub>w</sub> = 1.0 × 10<sup>-14</sup>, regardless of
Q65: Which of the following will be diamagnetic?<br>A)
Q68: When an atom is represented by the
Q68: A buffer is prepared by adding 150
Q71: Identify the missing species in the following
Q82: A mixture of 0.600 mol of bromine