The temperature at which the following process reaches equilibrium at 1.0 atm is the normal melting point for phosphoric acid. H3PO4(s)  H3PO4(l)
H3PO4(l)
Use the following thermodynamic information at 298 K to determine this temperature.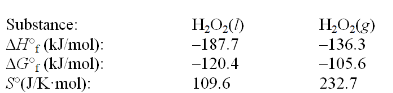
Definitions:
George H.W. Bush
The 41st President of the United States (1989–1993), known for his leadership during the Gulf War and his significant impact on foreign policy.
Categorical Data
Data that can be divided into specific groups or categories according to particular qualitative properties.
Quantitative Data
Quantitative data refers to information that can be measured and written down with numbers, expressing quantities, lengths, or sizes.
Census
A survey to collect data on the entire population.
Q21: A battery is considered "dead" when<br>A) <img
Q46: Which of the following pairs has the
Q53: When a weak acid is titrated with
Q62: Which of the following types of radioactive
Q63: The decomposition of dinitrogen pentaoxide to nitrogen
Q68: Ammonia will react with oxygen in the
Q79: An acetate buffer has a pH of
Q81: Sodium peroxide is an oxidizer used to
Q89: Which of the following aqueous liquids will
Q94: What is the chemical symbol for the