Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 25°C. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 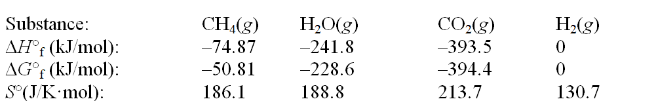
Definitions:
ICD-10-CM
The International Classification of Diseases, Tenth Revision, Clinical Modification; a system used by health care providers to classify and code all diagnoses, symptoms, and procedures recorded in conjunction with hospital care in the United States.
Diagnoses
The identification of the nature and cause of a health condition through examination of symptoms and tests.
Alphabetic Index Guidance
Assistance or instructions on how to use or navigate an index sorted by alphabetical order, typically found in reference books or databases.
ICD-10-CM General Note
Guidelines for coding and classification of diseases in a standardized manner as per the International Classification of Diseases, Tenth Revision, Clinical Modification.
Q19: In the electrolysis of aqueous potassium nitrate
Q35: The solubility of magnesium phosphate is 2.27
Q45: Calculate ΔG° for the reaction of iron(II)
Q52: Select the nuclide that completes the following
Q61: In a Millikan oil-drop experiment, the charges
Q61: Select the nuclide that completes the following
Q68: A buffer is prepared by adding 150
Q82: The term microstate refers to the energy
Q93: You need to use KH<sub>2</sub>PO<sub>4</sub> and K<sub>2</sub>HPO<sub>4</sub>
Q95: Tetrasulfur dinitride decomposes explosively when heated. What