Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 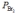 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
Definitions:
Finite Capacity Scheduling
Finite Capacity Scheduling is a planning method that takes into account the limited resources (such as machinery or workforce) available to complete tasks within a given timeframe.
Instantaneous Change
A rapid or sudden shift in conditions, values, or states, often used in mathematics and physics to describe changes occurring at a specific point in time.
Q4: A sample of a monoprotic acid (HA)
Q14: Carbon monoxide and chlorine combine in an
Q17: A buffer is prepared by adding 100
Q25: What is the name of the acid
Q35: Hexachlorophene is used as a disinfectant in
Q44: Which of the following substances has the
Q45: Calculate ΔG° for the reaction of iron(II)
Q74: Oxygen gas (O<sub>2</sub>) is paramagnetic, but ozone
Q80: A solution of ethanol (C<sub>2</sub>H<sub>6</sub>O) in water
Q99: Esters can be formed by the dehydration-condensation